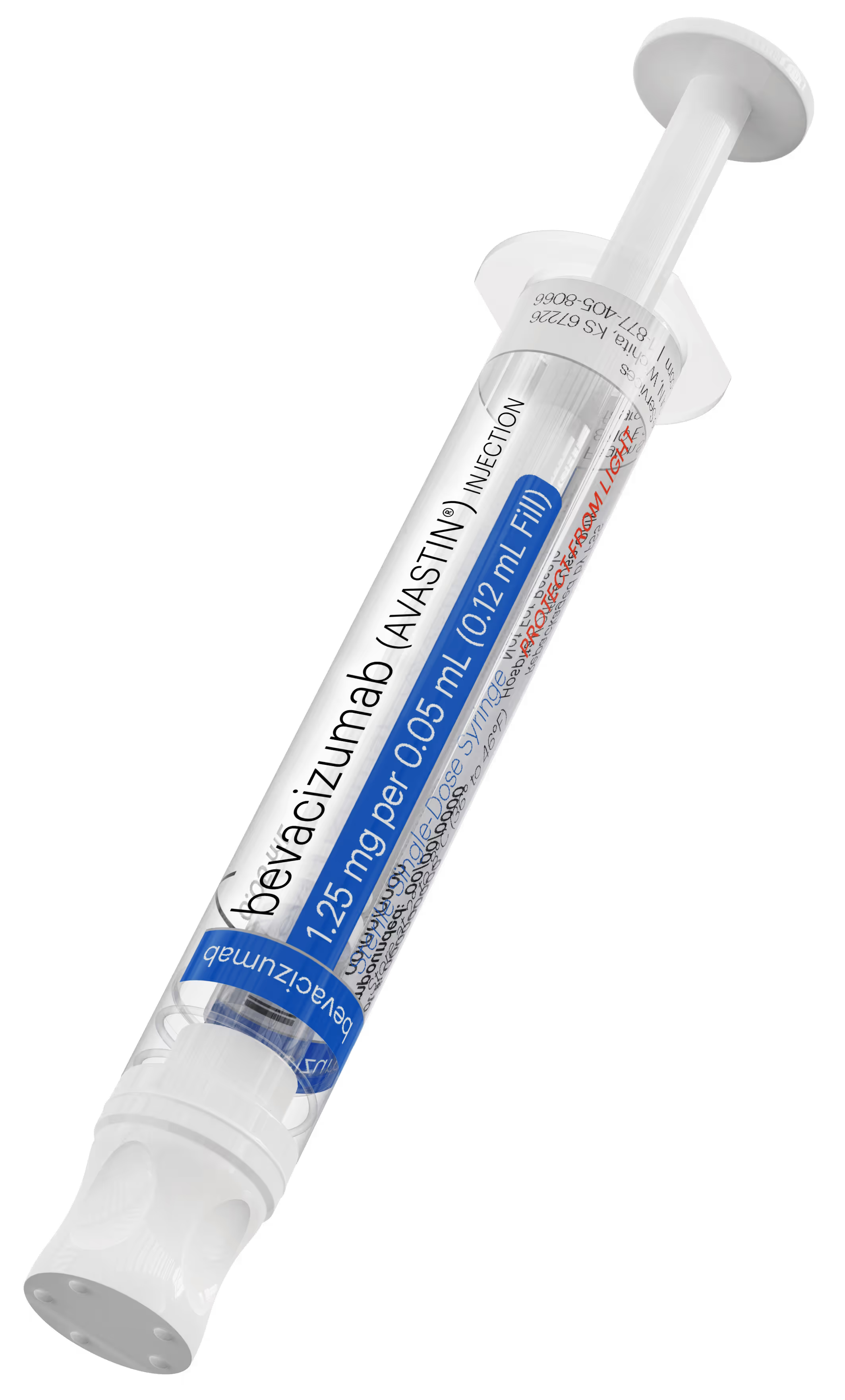
Revolutionary repackaged bevacizumab (Avastin®) injection from a 503B outsourcing facility
The innovative new bevacizumab (Avastin®) filled Daikyo Crystal Zenith® syringe, available from Fagron Sterile Services US (FSS), is produced with state-of-the-art Groninger automation through cGMP compliant operations to deliver a groundbreaking advancement.
You care about your patients; they trust your judgment.
“Ophthalmic injections represent one of the highest risk categories in all of injectable drug delivery. These are often repeat injections into the confined space of the eye, ultimately with the patient’s vision at stake. Historically, not enough has been done to manage these risks, especially as they relate to the syringe used to store or deliver the drug. We now have an option to reduce the risks that inadequate syringe systems can introduce.”
Innovation from process to product
This revolutionary new product sets a new standard for high-quality sterile compounding of repackaged bevacizumab (Avastin®).
The collaboration between FSS and West provides a pharmaceutically elegant, specially created biologics container, filled by an automated process; eliminating the reliance on human contact and manual syringe manipulation to prepare a sterile product.

FDA / DEA Registered & Inspected
Compliance with FDA, DEA, cGMP, GDP and cGLP regulations and guidance ensure our process is sound and supports patient safety
cGMP Compliant
Three decades of experience employing subject matter expertise with 21 CFR Parts 210 and 211 produces quality you can trust
ISO 5 environments
16 independent aseptic processing environments with dedicated HVAC systems, automation and depyrogenation technology
Committed Supply
The medication you want, when you need it — period. Talk with an Account Representative today about our Instant Ship program
100%Tested
All batches must successfully pass tests for sterility, particulate matter and potency prior to being released

Industry Leading Automation
Groninger Filling Machines are recognized as a global leader in syringe processing with nested pre-sterilized syringes preventing possibilities of microbiological contamination.
Groninger uses a Flexicon high-accuracy peristaltic pump providing 3% filling accuracy. The filling machine is installed inside a Restricted Access Barrier System (RABS) providing an Aseptic Processing environment reducing or eliminating interventions (human intervention) into critical zones of the filling process.
The processing suite is equipped with its own air handling unit (AHU), independent high-quality filtration and continuous environmental monitoring. The RABS system provides unidirectional airflow providing a Class A (ISO-5) environment pushing air though terminal Ultra-Low Particulate Air (ULPA) filters removing 99.9995% of particles down to 0.1 (µm) micrometers.
The Groninger filling machine and RABS system are fully validated per FDA 21 Code of Federal Regulations (CFR).
The CFR is a codification of the general and permanent rules published in the Federal register by the Executive departments and agencies of the Federal Government. Title 21 of the CFR is reserved for rules of the Food and Drug Administration (FDA).
The equipment uses all presterilized components which are loaded, filled, and finished in the ISO-5 environment.
Presterilized trays are loaded in the fill machine, a finite amount of sterilized fluid is dispensed into each syringe via peristaltic pumps and two needles.
The syringes are then stoppered and removed from the machine for post-production quality inspections.

Daikyo Crystal Zenith® Cyclic Olefin Polymer Syringe Features
Daikyo Crystal Zenith® Cyclic Olefin Polymer barrel,
Extended shelf life
Flurotec® barrier film on the plunger and tip cap,
Highly consistent syringe functional forces and a larger, ergonomic outer diameter of the barrel,

Syringe Dimension Specification
Length from tip cap to end of plunger rod
Width of the barrel for each individual syringe chamber
Syringe flange (widest point of the syringe)
From tip cap to syringe flange
Avastin® Dosing Accuracy
Administration subjectivity can result in large variations of a drug administered to the patient. Studies have demonstrated that dosing errors can be as high as 50% with either over or under dosing depending on the syringe3.
Dosing accuracy, or the amount of drug administered to the patient, is of critical importance to support patient safety and successful clinical outcomes. Without an accurate, constant volume of medicine, the quality of treatment may vary. This is particularly important in Avastin® injections where the volume of drug administered is so small.
The new presentation from FSS addresses these concerns and brings to market the first highly accurate, ergonomic container, intended for Avastin® administration.
Did you know?
Together we create the future of personalizing medicine.
Over the past 34 years, Fagron has earned the trust of pharmacists in communities across more than 30 countries by prioritizing transparency and delivering high-quality medications supporting patient care.
Fagron Sterile Services US (FSS) is a DEA and FDA-registered and inspected supplier, providing a broad portfolio of high-quality sterile medication and expertise in pharmaceutical manufacturing, repackaging, patient safety, regulatory guidance and pharmacy.
268K+ ft²
Capacity across 3 state-of-the art operational facilities
100%
of batches tested for sterility, particulate matter and potency to support patient safety
Diverse product portfolio
Supporting providers and the patients you serve across the continuum of care
Instant ship program
Medications when you need them the most, in-stock, ready to ship

Talk to a representative to pre-order today
FSS’ Avastin demonstrates a commitment to innovative solutions and partnership with customers to provide a reliable supply of high-quality medications for their patient-care needs.
Globally and vertically integrated operations
Fagron’s 4k+ employees serve 30+ countries from 70+ facilities
Proactively supporting supply chain resiliency
Fagron’s global team of 100 supply chain experts leverage 3k+ suppliers
Diverse portfolio across the continuum of care
FSS supports patient care from the delivery room to the O.R.
including clinics
1. Reference
2. BD Letter to Customers product information June 14, 2018
3. Avastin | Weill Cornell Medicine
4. Genentech in Competition With Itself on Eye Drug
5. Rosenfeld PJ, Windsor MA, Feuer WJ, Sun SJJ, Frick KD, Swanson EA, Huang D. Estimating Medicare and Patient Savings from the Use of Bevacizumab for the Treatment of Exudative Age-related Macular Degeneration. American Journal of Ophthalmology. July 2018; 191:135-139
6. Medicare Part B Drug Spending Dashboard
7. Estimating Medicare and Patient Savings From the Use of Bevacizumab for the Treatment of Exudative Age-related Macular DegenerationRosenfeld, Philip J. et al. American Journal of Ophthalmology, Volume 191, 135 – 139
Crystal Zenith® and Daikyo Flurotec® are registered trademarks of Daikyo Seiko, Ltd. Avastin® is a registered trademark of Genentech, Inc.

